New Customers Sepcial: Get 15% off, use the code "NEW15" at checkout. Shop Now!
Human α-synuclein monomer Protein (Type I)
A full-length, untagged recombinant Human Alpha-Synuclein monomer protein, biologically active, with Uniprot: P37840. This Recombinant Human Alpha-Synuclein Monomer Protein is expressed in E. coli.
1. Neurodegenerative Disease Research:
Parkinson’s Disease: α-Synuclein monomer is widely used in studying the early stages of Parkinson's disease and other synucleinopathies. Understanding how the monomeric form of the protein behaves and its potential to aggregate into toxic oligomers and fibrils provides insights into disease mechanisms and helps identify targets for early intervention.
2. Modeling Protein Aggregation:
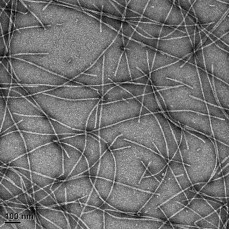
Researchers use recombinant human α-synuclein monomer to study protein misfolding and aggregation. These studies help explore how α-synuclein transitions from its functional monomeric form to pathological aggregates that form Lewy bodies, a hallmark of Parkinson's disease.
3. Drug Discovery and Screening:
Target for Drug Development: α-Synuclein monomer is a critical target in the development of therapeutics aimed at inhibiting or preventing its aggregation. Screening for small molecules, peptides, or antibodies that can prevent the aggregation of α-synuclein is an active area of research for Parkinson’s disease and other synucleinopathies.
4. In vitro Assays:
The monomer is used in high-throughput screening assays to identify potential compounds that could prevent α-synuclein aggregation or reduce existing aggregates in neurons or other cellular models.
5. Biomarker Discovery:
α-Synuclein monomer is studied as a biomarker for neurodegenerative diseases. Its misfolding and aggregation are closely linked to disease onset and progression. By studying its levels, forms, and aggregation state in biological fluids like blood or cerebrospinal fluid (CSF), researchers can develop methods for early diagnosis and monitoring disease progression.
6. Protein-Protein Interaction Studies:
Binding and Interaction Studies: The monomeric form of α-synuclein is used to study its interactions with other proteins and lipids, particularly in relation to synaptic vesicle function. Researchers use surface plasmon resonance (SPR) or isothermal titration calorimetry (ITC) to identify binding partners that influence its function, aggregation, or cellular activity.
7. Chaperone Function:
Investigating the interaction of α-synuclein monomer with molecular chaperones or other cellular proteins can help elucidate its role in protein quality control and cellular stress response mechanisms.
8. Vaccine and Immunotherapy Development:
Immunization: Researchers are exploring the use of α-synuclein monomer in the development of vaccines that aim to trigger an immune response against aggregated α-synuclein, preventing or clearing toxic aggregates. Immunotherapies targeting α-synuclein are under investigation for their potential to reduce or halt the progression of Parkinson's disease.
9. Monoclonal Antibodies:
The monomer is used to develop monoclonal antibodies that can specifically bind to α-synuclein aggregates or prevent its aggregation in the brain.
10. Cellular and Structural Biology:
Membrane Interaction Studies: The monomer is used to investigate how α-synuclein interacts with lipid membranes, which is essential for understanding its role in synaptic vesicle formation and neurotransmitter release. This helps clarify how it influences synaptic activity and its potential to cause neuronal dysfunction.
11. Structural Characterization:
Structural biologists use α-synuclein monomer to study its conformational flexibility using techniques such as NMR (nuclear magnetic resonance) or X-ray crystallography. These studies provide insights into the protein's ability to adopt different structures and how its misfolding and aggregation contribute to disease.
12. Gene Therapy and RNA Interference:
Gene Silencing: The monomer form is used in studies that aim to explore RNA interference (RNAi) or CRISPR/Cas9 strategies to reduce or knock out α-synuclein expression in cells or animal models. This research can help determine if reducing the levels of α-synuclein can prevent or mitigate neurodegeneration in Parkinson's disease.
TDD-1002-product-datasheet
Human α-synuclein monomer Protein (Type I).pdf
| Chemical | |
|---|---|
| Endotoxin | 1.0 EU per μg protein as determined by the LAL method |
| Purity | >96% SDS-PAGE; ≥ 98 % as determined by SEC |
| Biological | |
| Application | WB, SDS-PAGE, In vivo assay, In vitro assay |
| Biologically Active | Yes |
| Expression System | Escherichia coli |
| Organism | Homo Sapiens, Human |
| Protein Construction | Human SNCA (Uniprot: P37840) (Met1-Ala140) expressed no tag |
| Protein Type | Recommbinant, Wild Type |
| Shipping and Handling | |
| Shipping Condition | Dry Ice |
| Storage Buffer | 20 mM Tris-HCl, pH 8.0, 150 mM NaCl |
| Storage Temperature | -80℃ for long term storage; avoid freeze / thaw cycle |